Abstract:
Today, many Flow Sciences customers must safely handle powdery materials. The reasons for containing such material cover a robust range of applications:
- Mixing, blending, and dilution of active pharmaceutical ingredients. (API’s)
- Dispensing, weighing, and packaging dosing quantities of active pharmaceutical ingredients
- Quality control sampling of incoming bulk quantities of solid fillers or API’s
- Grinding, milling, or otherwise changing the texture of raw pharmaceutical ingredients
Recently, pharmaceutical facilities have seen an increase in new API’s. These newer powerful drug ingredients have reached a potency where containment equipment requirements have reached a much more demanding level 3.
Such containment devices usually include one or more of these attributes:
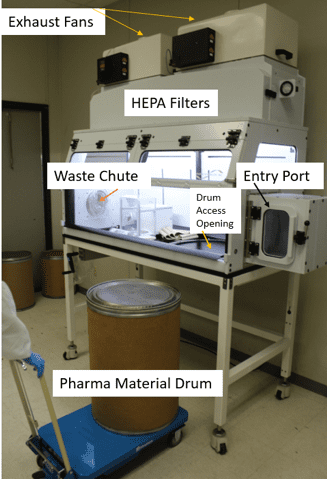
- A drum access opening in the worktop
- Some type of waste chute or other means for removal of contaminated materials from earlier procedures
- A fan/filtration system, typically with bag-in bag-out HEPA filters
- The containment capacity to keep room levels of materials being processed at or below 1mg – 50ng per cubic meter.
- A highly trained worker, armed with carefully-written manuals and SOP’s
The writer will flesh out some of the applications for such units and highlight newer products available to face the new challenges of active pharmaceutical ingredients.
Factors Needing Control in Powder Containment:
Working with powders can present unique containment challenges:
First, the need to bring kilogram quantities of compound into an environment with a microbalance places in close proximity objects that seldom see each in any other analytical context. We already know this “meeting” has to happen, particularly in weighing or compounding environments. The ultimate mandate is that this meeting must smooth the awkwardness of Goliath, while preserving the accuracy of David!
The correct powder containment device can accomplish this feat. Sufficient airflow keeps powders contained, while the non-turbulent flow isolates one process from other materials in the same workspace. While all this is happening, the device provides an environment with virtually no turbulence or vibration impact on balance accuracy.
Flow Sciences technologies for doing all this have a long history in lab design and the efficiencies of such systems continue to improve4.
Powders spread inside containment spaces not only because of air currents, but also due to the electrostatic properties of the powders themselves. Powders are typically charged in turbulent airflow. They “stick” to surfaces and adhere to the operator’s hands (figures 4 below).


Figures 4: UV Luminescent Powder Shows Possible Electrostatic Transport of Powder. Note operator’s gloves!
Researchers must be able to police the containment work area, leaving gloves and equipment behind as they withdraw their hands from a procedure. We should always preemptively collect and isolate such waste inside the device. Waste chutes, transfer ports, bagging, and the like are all ways to retain and control contamination inside devices which contain powders.
To process with high purity, intelligent airflow design, built-in solutions for debris collection and isolation, and worker training about SOP’s are all important. The following six examples of bulk powder devices illustrate how many new types of containment structures achieve very good results.
Examples of Powder Applications and Successful Containment:
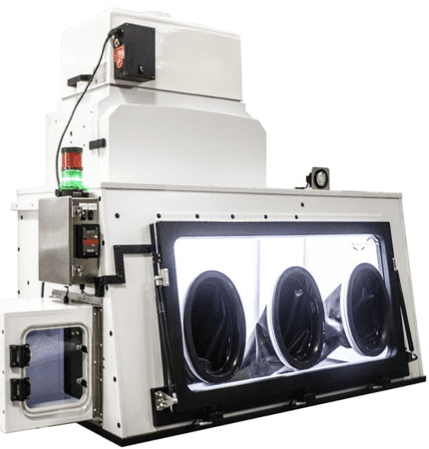
1) The EHP: A Hybrid Isolator with Many Available Options:
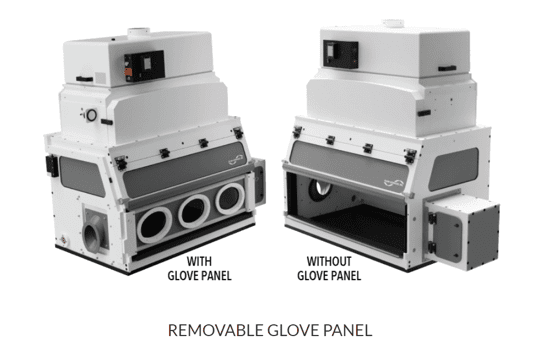
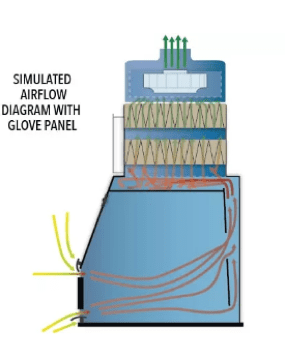

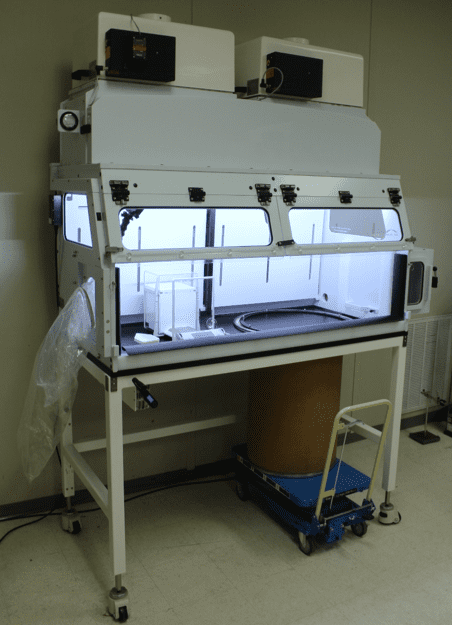
Fig 7: EHP used to weigh powder; rubber seal around drum;
vibration-isolated fan, and stable scale environment
In summary, the FSI EHP Hybrid Isolator can handle kilo-sized quantities of powder for weighing and compounding applications. The unit is also suitable for custom compounding pharmaceuticals or weighing quantities of unique materials into dose quantities. The standard EHP containment device can do all this with the proper accessories. It has all the supporting attributes set forth in the abstract and illustrated in Figures 5 through 8.FC
Such a device contains weighing operations. Batching and dosage may proceed with products then transferred to a port for removal. Mixing pre-weighed ingredients for pharmaceutical use is also possible with the EHP unit. The more complex the application, however, the larger the device may become, allowing more equipment and processes to be undertaken simultaneously.
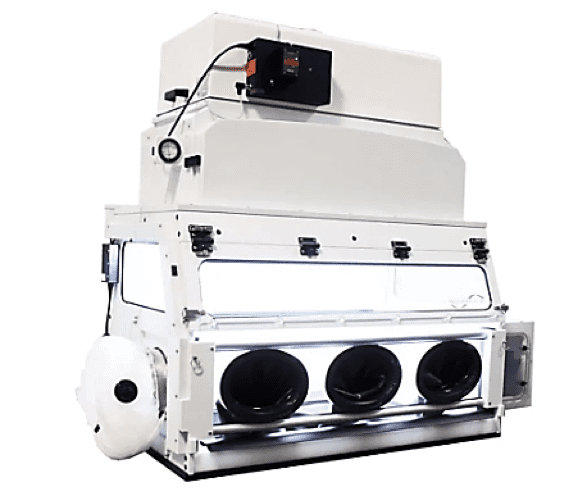
2) The Glovebox Workstation for LFBC (Lateral Flow Bio Containment Isolator)
The LFBC Isolator permits handling and manipulation of API (Active Pharmaceutical Ingredients) and HPAPIs (Highly Potent Active Pharmaceutical Ingredients) where personnel protection and product purity are required.
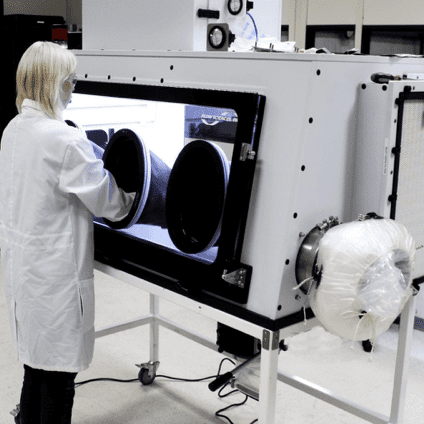
The LBFC very effectively isolates highly potent pharmaceuticals from the room environment using a lateral flow shown in Fig, 11 below. This unit can contain to nanogram levels and from OEB 3-5. Consult FSI about your application, as containment levels can vary based on application and quantity of material processed.
This cabinet is also a Class 1 Biosafety unit certified using ASHRAE 110-2016. Generally usable in BSL class 1, 2, or 3 environments. Provides personnel protection, not product protection.

3) Specialized Compounding, Liquid Dose Preparation from Bulk Powder:
The custom bulk powder unit shown below represents a more complex customer application. Addition of a measured amount of dry powder to a liquid in a mixing vessel on the right hand side to produce a liquid suitable for IV use.
Designed for personal protection, this unit has added features to facilitate working with powders using a Mettler Toledo XPE-10015 balance and an IKA mixer stand with the vessel top penetrating through the port on the right side of the worktop. This table section contains a black under-counter cabinet with a multiple height slide-out shelf for different size mixing vessels. Viewing panels are transparent acrylic.
Other features include energy-efficient LED lighting, black phenolic base, left side powder drum cutout, top-mount fans, dual HEPA filters with bag- in bag-out filter system (BIBO), iris ports on both sides, minihelic static pressure gauge, and a table with lock-down casters.

4) Sieving and Drug Compounding Bulk Powder Containment:

This Bulk Powder Station has dual, Bag-In/Bag-Out fan/HEPA filtration systems. Designed to vent and filter small batch sieving machine dust, the enclosure is equipped with a stainless steel lift cart and fitted with 20” bulk powder cutout for loading drums through the work top access hole. Hinged front and lower doors allow for additional equipment and samples. Airfoils, rear plenums, and top-mounted fans maintain laminar airflow across the workspace ensuring minimal exposure to hazardous powder APIs. The polypropylene superstructure is fitted with acrylic panels and built-in LED lighting.
5) Very large quantity unit:

of the unit horizontally.
This large quantity unit features three top-mounted HEPA filtration systems. Designed for personnel protection while working with powder substances, the unit also houses a related balance application. The superstructure is acrylic with hinged upper access doors. A waste chute is located on the right side wall.
6) Animal Waste Processing:
In some cases, the “powder” being processed is waste, not ultra-pure ingredients. The stainless steel exhaust hood shown below offers our customers an isolated environment where animal cages are cleaned. Crushed clay absorbent is disposed of down the circular chute into a waste container below.
The coved 300 series stainless steel liner is easy to disinfect after use. Containment testing demonstrated isolation of waste contaminants inside.

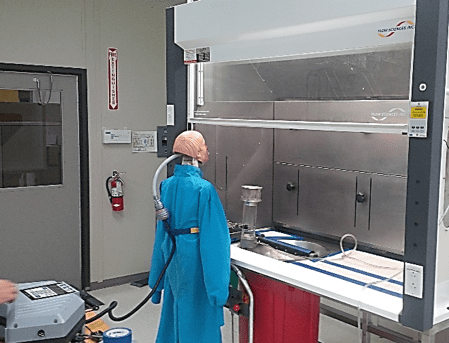
Fig. 10: A Bulk Waste Unit, ASHRAE 110 tested, handles cage cleaning byproducts!
7) Stainless Steel Bulk Powder Units
Customers who have a need for very clean interior surfaces or effective contamination removal frequently opt for 300 grade stainless steel interiors. The powder device shown below has been tested and validated per end user requirements before leaving the FSI manufacturing facility.

Are Devices Like These Right for Your Application?
There are, as we have seen, many types of powder containment devices designed to house a variety of different processes. Containment (personal protection) depends upon good and useful airflow, which keeps powders away from workers in the lab space, but there is so much more!
Because of electrostatics, workers should follow guidelines for properly using the opening. They must never remove an un-bagged object from the containment area. Since gloved hands can inadvertently bring hazardous powders out of containment areas, gloves and expendables should be kept under containment inside the containment area and be disposed using a waste chute. Ultimately, the work area must be clean after the process is complete. Otherwise serious contamination will occur. If the bulk powder containment device has no waste chute or portal, cleanup is virtually impossible. Beware of poorly designed units that allow no disposal routes from inside the containment area.
Experience with such units has shown that process-specific testing is necessary to demonstrate containment using proper technique by end-users. For this reason, ASHRAE 110-2016 and surrogate powder testing reports using the ISPE APCPPE Guideline1 must be part of the equipment validation procedure. Flow Sciences standard and custom containment equipment always come with documentation similar to that shown here!

All Flow Sciences, standard products easily pass both tests. Custom bulk powder containment units are tested with ASHRAE 110-2016 and the ISPE APCPPE Surrogate Powder Guideline2 using the customers’ procedures inside the unit. One such published study showed great containment for a standard Flow Sciences chemical fume hood using both containment tests. Results for surrogate powder and solvent vapor containment showed undetectable powder levels in the lab work area after significant interior powder and solvent exposure was created during the test. 2
In addition, manuals for all our bulk powder units contain complete instructions for coupling and decoupling large ingredient drums, along with more general techniques necessary for effective containment of materials within the work area.

Conclusions:
- Powder units should allow direct access to large quantities of powdered pharmaceutical materials while providing superior personal protection. Lab containment equipment should not expose workers to mixing, weighing, grinding, sieving, compounding, or other processes within an inadequate containment device.
- We should test containment units against contamination release using both ASHRAE 110-2016 and surrogate powder testing using the ISPE APCPPE Guidelines and processes relevant to the specified application.
- Powders differ from gases in that they will typically stick to surfaces, including the researcher’s hands and lab coat! Any successful test report by a manufacturer of such equipment should also include a review of cleaning and housekeeping procedures designed to prevent static transmittance of the relevant materials.
Footnotes:
- Assessing the Particulate Containment Performance of Pharmaceutical Equipment, Second Edition, 2012, International Society for Pharmaceutical Engineering
- Evaluating a Fume Hood for Containment of Solids, Liquids, and Vapors Using ASHRAE 110, HAM, and ISPE Methods, Goodman and Haugen, 2019, available from Flow Sciences, Leland NC
- Cyto Solutions, Engineering controls for the Containment of Cytotoxic Materials, Flow Sciences 2019, https://flowsciences.com/containment-engineering-controls-for-cytotxic-hpapi-adc/
- Red Lights and Green Lights-The Keys to Superior Containment, Robert Haugen, 2018, Flow Sciences, https://flowsciences.com/the-keys-to-superior-containment-in-compounding-applications/

